Cook Medical has reached a key milestone in the development of its next-generation endovascular technology, announcing the completion of patient enrollment in the global clinical study of its Zenith Fenestrated+ Endovascular Graft (ZFEN+). The company confirmed that the final patient has been treated, marking the transition from recruitment to data analysis in a pivotal trial that could shape the future of complex aortic aneurysm repair.
The study, conducted under an FDA investigational device exemption (IDE) granted in June 2023, is designed to evaluate the safety and effectiveness of ZFEN+ when used in combination with:
The investigational Zenith Universal Distal Body 2.0 graft (Unibody2).
The investigational Bentley BeGraft balloon-expandable fenestrated endovascular aneurysm repair (FEVAR) bridging stent graft system.
The commercially available Zenith Spiral-Z AAA iliac leg graft (ZSLE).
By closing enrollment, Cook Medical is now positioned to analyze outcomes data that will be critical for regulatory submission and eventual commercialization.
Extending the reach of fenestrated grafts
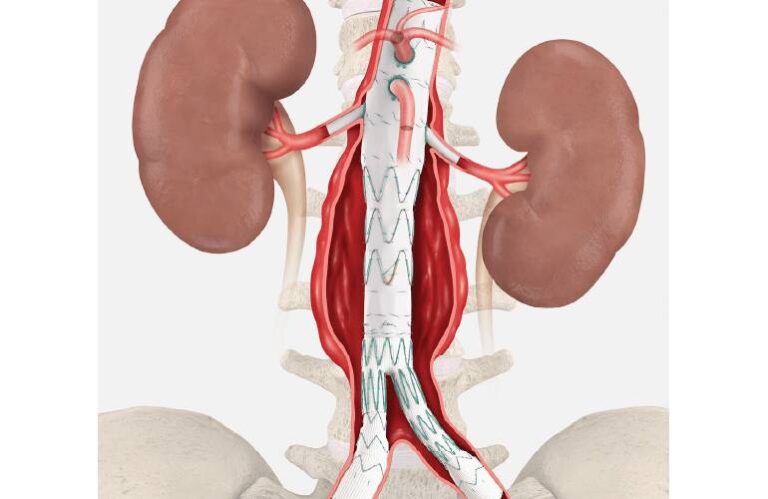
ZFEN+ builds upon the foundation of Cook’s commercially available ZFEN AAA endovascular graft, which has been widely used in abdominal aortic aneurysm (AAA) repair. The “+” iteration expands the proximal margin of treatable aneurysmal disease, allowing physicians to address more complex anatomies involving one or more of the major visceral arteries.
The device incorporates up to five precisely located fenestrations to accommodate visceral vessels, with the option of combining fenestrations and a scallop—up to a maximum of five total openings. This high level of customization allows physicians to tailor each graft to the patient’s anatomy, enabling endovascular repair in cases that historically required open surgical intervention.
By maximizing the seal zone to exclude the aneurysm while preserving blood flow to critical vessels, ZFEN+ addresses one of the greatest challenges in complex aortic aneurysm treatment: balancing durability with patient safety.
Clinical significance
Aortic aneurysms—particularly those involving visceral arteries—are among the most challenging conditions in vascular surgery. Traditional open repair carries significant risks, especially in older or comorbid patients. Fenestrated endovascular grafts offer a minimally invasive alternative, but their use has been limited to specialized centers and select anatomies.
ZFEN+ could expand access by enabling more patients to be treated with an endovascular-first approach. If successful, the system may become a cornerstone in managing abdominal and thoracoabdominal aneurysms, reducing the need for invasive open procedures and their associated complications.
Leadership perspective
“Now that enrollment is complete, we are one step closer to understanding the outcomes of patients treated with ZFEN+ and, ultimately, to advancing care for patients with complex abdominal and thoracoabdominal aneurysms,” said Johnny LeBlanc, director, product management, Aortic at Cook Medical.
He credited the milestone to the collaboration between Cook’s research team and clinical partners:
“Reaching this milestone is a direct reflection of the dedication shown by our investigator partners, study coordinators, and—most importantly—the patients who supported the study with their time and trust. We look forward to analyzing the data and sharing the results with the clinical community.”
A step forward in aortic innovation
The completion of patient enrollment represents not just progress for Cook Medical but also for the broader field of aortic repair. With an increasing number of patients diagnosed with complex AAAs and thoracoabdominal aneurysms, the demand for customizable, minimally invasive solutions continues to grow.
ZFEN+’s ability to accommodate multiple visceral arteries with precision-engineered fenestrations highlights the industry’s shift toward personalized device design, where grafts are tailored to match patient-specific anatomy. This evolution has the potential to improve outcomes, shorten recovery times, and expand treatment eligibility to patients who previously lacked viable options.
As Cook Medical prepares to analyze data from this pivotal trial, the results will be closely watched by clinicians, regulators, and competitors alike. The findings could pave the way for regulatory approval and broader adoption, positioning Cook at the forefront of endovascular solutions for complex aneurysm repair.
Competitive landscape: Cook Medical’s position in complex aortic repair
The completion of patient enrollment in Cook Medical’s pivotal ZFEN+ study marks a turning point not only for the company but also for the competitive landscape in fenestrated endovascular aneurysm repair (FEVAR). Complex aortic repair has become a battleground for MedTech innovators, with companies vying to provide surgeons the most versatile, durable, and patient-specific solutions. Cook’s long history in fenestrated technology and its new push with ZFEN+ place it in a strong position against rivals like Medtronic and W. L. Gore & Associates.
Cook Medical: the pioneer in fenestrated repair
Cook Medical has long been recognized as a pioneer in endovascular aneurysm repair (EVAR). Its Zenith platform was among the first to bring modular, fenestrated solutions to market, allowing physicians to address challenging anatomies without open surgery. Over the years, Cook has invested in customization, modular graft designs, and physician training, establishing itself as a reference player in complex AAA and thoracoabdominal aneurysm management.
ZFEN+ extends this heritage, offering:
More proximal sealing zones for complex aneurysms.
Up to five fenestrations and one scallop, adaptable to patient-specific anatomies.
Integration with complementary grafts (Unibody2, BeGraft, ZSLE), creating a comprehensive ecosystem.
This ecosystem approach could give Cook a strategic advantage as physicians increasingly seek customizable solutions that can be tailored for individual patients.
Medtronic: pushing modularity and off-the-shelf designs
Medtronic, a leading competitor, has focused on modular stent graft designs that emphasize ease of use and broader anatomical applicability. Its Valiant Navion (since withdrawn for safety issues) and Endurant II/IIs platforms illustrate Medtronic’s push into standard EVAR and thoracic EVAR (TEVAR).
In the FEVAR segment, Medtronic continues to innovate around physician-modified devices and early-stage fenestrated platforms, aiming to expand beyond standard infrarenal aneurysm repair. Medtronic’s global reach, brand strength, and large procedural footprint give it commercial clout, but Cook’s deep fenestration experience allows it to compete directly in the most complex cases.
W. L. Gore: focusing on simplicity and conformability
W. L. Gore & Associates has pursued a different angle with its Excluder stent graft system, known for its conformability and ease of implantation. Gore’s efforts in branched and fenestrated grafts, particularly with investigational designs for thoracoabdominal aneurysms, target physicians who prefer streamlined device delivery systems.
Gore’s approach appeals to centers performing higher volumes of straightforward cases, but when it comes to highly complex aneurysms requiring multiple fenestrations or scallops, Cook’s ZFEN+ offers more tailored versatility.
Market dynamics: growing demand for complex aneurysm solutions
Globally, the market for aortic aneurysm repair is growing at an estimated 5–6% CAGR, with complex AAA and thoracoabdominal cases representing a rising share of procedures. Key drivers include:
Aging populations in North America, Europe, and Asia-Pacific.
Increased detection through advanced imaging.
Shift toward minimally invasive procedures, reducing reliance on open repair.
Historically, many patients with juxtarenal or thoracoabdominal aneurysms were excluded from EVAR due to anatomical challenges. Devices like ZFEN+ directly target this underserved population, expanding the treatable pool and potentially improving outcomes.
Competitive implications for ZFEN+
Cook’s ZFEN+ could reshape the competitive balance by:
Expanding the scope of EVAR – Allowing endovascular treatment in patients who previously required open repair.
Enhancing physician adoption – By offering customizable fenestrations, Cook strengthens its reputation as the “surgeon’s partner” in complex aortic cases.
Building an ecosystem – With Unibody2, BeGraft, and ZSLE integration, ZFEN+ creates a comprehensive suite that competitors may find difficult to match.
Setting a clinical benchmark – If the pivotal trial demonstrates safety and durability, ZFEN+ could become the reference device for FEVAR in the U.S. and abroad.
Challenges ahead
Despite its strong positioning, Cook faces hurdles:
Time to approval: The trial must complete follow-up and data analysis before submission, likely delaying widespread U.S. adoption until later this decade.
Competitive pressure: Medtronic and Gore may accelerate their fenestrated or branched pipeline programs in response.
Training and expertise: Fenestrated repair remains technically demanding; widespread adoption depends on physician training and institutional support.
Nonetheless, the completion of patient enrollment signals momentum and positions Cook as a frontrunner to define the next chapter in complex EVAR.
Powered by Froala Editor