Airiver Medical, a Brooklyn Park, Minnesota-based MedTech innovator, announced it has received FDA investigational device exemption (IDE) approval for its pulmonary drug-coated balloon (DCB) system. The IDE—the company’s first—clears the way for a pivotal clinical trial evaluating the device’s safety and efficacy in treating central airway stenosis, a debilitating condition with limited treatment options.
Addressing an unmet need in airway care
Central airway stenosis refers to narrowing of the trachea or bronchi, often resulting from intubation, tracheostomy, tuberculosis, lung transplantation, or other underlying conditions. Left untreated, the narrowing can cause severe breathing difficulties, recurring infections, or even respiratory failure. Current treatment options are limited to mechanical interventions such as balloon dilation, stenting, or repeated procedures, none of which reliably prevent recurrence.
Each year in the U.S., physicians perform an estimated 100,000 tracheo-bronchial stenting and dilation procedures, reflecting the scale of the unmet need. Yet despite these interventions, recurrence remains common, forcing patients into repeated cycles of invasive treatment and undermining their quality of life.
Airiver aims to change that paradigm with its pulmonary DCB system.
Combining balloon dilation with targeted drug delivery
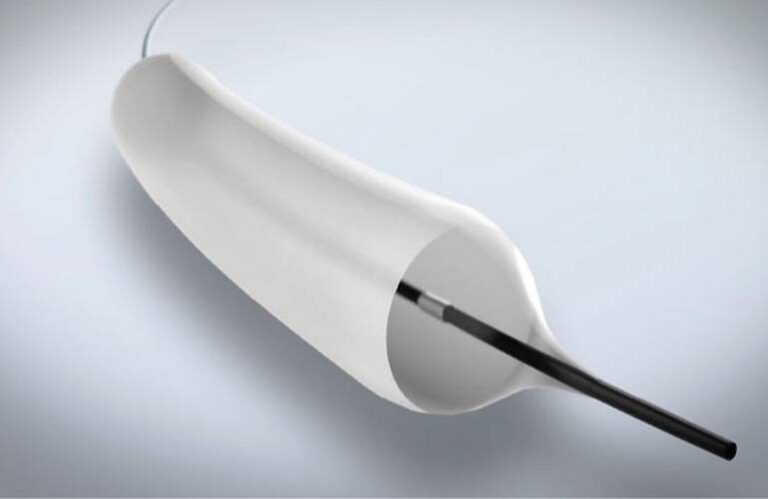
Airiver’s DCB technology is designed to do more than open the airway temporarily. While balloon dilation expands the stenotic segment mechanically, Airiver adds a proprietary paclitaxel-based coating that delivers localized therapeutic treatment directly to the diseased tissue.
The goal is twofold:
Immediate symptom relief by physically opening the airway.
Long-term durability by delivering antiproliferative drug therapy to reduce scarring, inflammation, and restenosis.
According to Airiver, the proprietary coating formulation ensures that paclitaxel is delivered only where needed—at the site of stenosis—while minimizing drug exposure to surrounding healthy tissue. This precision drug delivery could reduce recurrence rates and eliminate the need for frequent re-interventions.
The pivotal IDE trial
The newly approved IDE study will serve as the foundation for FDA regulatory submission and potential U.S. commercialization of Airiver’s pulmonary DCB. Key features of the trial include:
Enrollment of up to 200 patients diagnosed with central airway stenosis.
Nationwide participation, with multiple leading interventional pulmonology centers taking part.
Randomized comparison of the Airiver DCB versus bare balloon dilation, which currently represents the standard of care.
Safety and efficacy endpoints, focusing on recurrence rates, durability of airway opening, and improvements in patient quality of life.
By directly comparing the device against today’s gold standard, the study aims to provide definitive evidence of whether drug-coated balloon technology can deliver a step-change improvement in patient outcomes.
A breakthrough opportunity in airway intervention
While drug-coated balloons are well established in cardiovascular applications—such as treating coronary and peripheral artery disease—Airiver’s platform represents the first effort to apply DCB technology in the pulmonary space.
If successful, Airiver’s innovation could introduce a new minimally invasive, durable treatment option for central airway stenosis, transforming the care pathway for thousands of patients annually. Instead of relying on repeat dilations and stenting, patients could benefit from a single, drug-enhanced intervention with longer-lasting results.
Leadership perspective
Airiver’s founder, CEO, and chief technology officer Lixiao Wang framed the IDE approval as a pivotal milestone:
“As it stands, there is no optimal treatment of recurrent airway stenosis available as part of today’s treatment paradigm. Securing IDE approval for this study is extremely exciting because the Airiver DCB has the potential to establish a new minimally invasive and durable treatment option preventing recurrence for patients suffering from this serious condition, which has not yet been accomplished.”
Wang’s comments reflect the company’s broader ambition: to introduce a foundational technology platform that could extend into other airway and luminal applications over time, much as DCBs did in the cardiovascular field.
Strategic implications
With IDE approval secured, Airiver enters the clinical stage with momentum. The pivotal trial outcome will determine whether the company can progress toward FDA approval and commercialization in the U.S. Success could not only validate Airiver’s proprietary coating and pulmonary DCB approach but also open a new category in interventional pulmonology—one that combines mechanical and pharmacological therapies in a single procedure.
For physicians, this could mean fewer repeat procedures and more effective care. For patients, it could mean fewer hospital visits, longer-lasting symptom relief, and improved quality of life.
Competitive landscape: Airiver introduces drug-coated balloons to pulmonary care
Airiver Medical’s FDA IDE approval for its pulmonary drug-coated balloon (DCB) marks the arrival of a technology that could disrupt the current treatment paradigm for central airway stenosis (CAS). While balloon dilation and stenting remain the prevailing standards, their limitations create an opening for innovation. Airiver’s DCB introduces a drug-delivery approach into interventional pulmonology, a field that has not yet fully leveraged the drug-eluting or drug-coated strategies already common in cardiology.
Current standard of care: balloon dilation and stenting
Today, CAS is managed with a toolkit of mechanical solutions:
Balloon dilation: Widely performed to temporarily expand the narrowed airway. While effective in the short term, restenosis occurs frequently, requiring repeat interventions.
Airway stents: Typically silicone or self-expanding metallic stents (SEMS), sometimes polymer-covered. These maintain airway patency but often migrate, accumulate mucus, and incite inflammation.
Surgical approaches: Reserved for select patients, but invasive, high-risk, and not broadly applicable.
Despite these tools, recurrence remains high, and long-term durability is lacking. For patients, this often means repeated hospital visits, additional procedures, and compromised quality of life.
Airiver’s differentiator: targeted pharmacology
Airiver is positioning its pulmonary DCB as a hybrid solution: combining the immediate mechanical benefits of balloon dilation with the long-term biological benefits of localized paclitaxel delivery.
Paclitaxel, long used in cardiovascular stents and balloons, inhibits smooth muscle cell proliferation and scarring. By applying it directly to the airway lesion, Airiver seeks to prevent restenosis at the source.
What makes this approach particularly compelling is its local precision:
Localized drug delivery avoids systemic exposure and side effects.
Proprietary coating ensures drug is deposited only at the stenotic site.
Durable effect could reduce or eliminate the need for repeat dilations and stent placement.
If successful, this approach could shift CAS management from short-term palliation toward long-term disease control.
Competitive players in airway interventions
The pulmonary intervention market has traditionally been dominated by companies producing airway stents and dilation tools:
Boston Scientific: Offers the Ultraflex and Aero stents, widely used in malignant and benign airway obstructions.
Merit Medical: Manufactures the Endotek line, including silicone, metallic, and hybrid airway stents.
Micro-Tech Endoscopy: Strong in self-expanding stents, with growing penetration in U.S. and European markets.
Olympus and Cook Medical: Provide bronchoscopic dilation tools and stenting options but have not ventured into drug-based solutions.
None of these players currently offer a drug-coated or drug-eluting pulmonary product, leaving Airiver with a first-mover advantage. By introducing pharmacological therapy into airway stenosis, the company may redefine the competitive field entirely.
Lessons from cardiology: why DCBs work
Airiver’s strategy mirrors the trajectory of drug-coated balloons in cardiovascular care. In coronary and peripheral arteries, DCBs overcame the limitations of bare-metal stents and balloon angioplasty by reducing restenosis and improving durability without leaving a permanent implant.
That same principle—mechanical opening plus localized drug therapy—could now apply to the pulmonary space. If Airiver proves its concept clinically, pulmonology could follow a similar path to cardiology, with drug-delivery devices becoming the new gold standard.
Market opportunity
Approximately 100,000 tracheo-bronchial dilations and stent placements occur annually in the U.S., a sizable market for innovation. With high recurrence rates under the current standard of care, physicians and patients are eager for longer-lasting solutions.
If Airiver’s pulmonary DCB reduces reintervention rates:
Hospitals and payors benefit from fewer repeat procedures and reduced overall costs.
Physicians gain a more effective and predictable tool.
Patients experience better quality of life, with fewer hospitalizations and interventions.
This alignment of clinical benefit and economic efficiency could accelerate adoption, particularly as value-based healthcare frameworks increasingly reward durable outcomes.
Challenges ahead
Despite its promise, Airiver faces several hurdles:
Clinical validation: The pivotal IDE trial must show statistically significant superiority over bare balloon dilation.
Adoption inertia: Physicians accustomed to mechanical-only interventions may be cautious about adopting drug-based tools without long-term evidence.
Regulatory precedent: Being the first pulmonary DCB, Airiver will need to establish new regulatory and reimbursement frameworks.
Competitive responses: If successful, larger players like Boston Scientific or Merit could quickly enter the space with their own drug-based solutions.
Strategic positioning
By being first, Airiver has an opportunity to define a new treatment category in interventional pulmonology. If the pivotal trial succeeds, the company could not only secure FDA approval but also establish itself as a platform innovator, potentially expanding into adjacent luminal applications such as esophageal, gastrointestinal, or vascular DCBs.
For now, Airiver’s pulmonary DCB represents a bold step forward—an effort to bring the pharmacological sophistication of cardiovascular interventions into the pulmonary arena, with the goal of transforming outcomes for patients with central airway stenosis.
Powered by Froala Editor